Infections
Background Infection Rates in the MS Population1–3
- Patients with MS have a higher risk of infection and hospital admission rates for infection compared with the general population
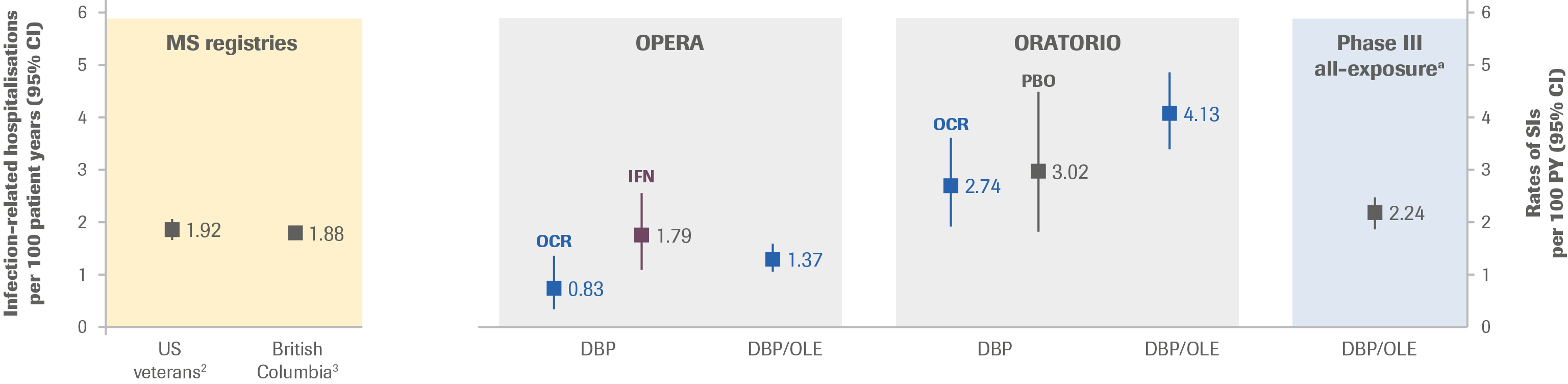
- A study assessing data from The Department of Veterans Affairs included 7,743 veterans with, and 30,972 without MS. Incidence rates (95% CI) for SI were 1.92 (1.76–2.08) per 100 PY for those with MS vs 1.03 (0.98–1.09) per 100 PY for those without MS
- A population-based study assessing data from British Columbia found that exposure to any DMT (7,682.1 PY) compared with no exposure (51,662.8 PY) was not associated with a significantly altered hazard for an infection-related hospitalisation (adjusted HR, 0.98; 95% CI, 0.77–1.26)
Clinical Trials (Controlled Treatment Period and Open-Label Extension)2–6
- In the controlled treatment period of the ocrelizumab Phase III clinical trials, infections were one of the most frequently reported AE
- In the Phase III trials, no increased risk of SI with ocrelizumab vs IFN β-1a or placebo was observed
- At approximately 6 study years of ocrelizumab exposure, the rate (95% CI) of SI was 2.24 (1.96–2.56) per 100 PY in the Phase III all-exposure population (DBP and OLE)
- The rate remained generally consistent with the rate observed at the primary analysis cut-off date and with rates of infection-related hospitalisation in real-world MS cohorts (Figure 1)
- The most common SIs were UTI, pneumonia and cellulitis
Figure 1: Incidence Rates of SI in Ocrelizumab Clinical Trials vs Infection-Related Hospitalisation from Select Registries per 100 PY
- As of January 2019, in the ocrelizumab all-exposure population (Phase II/III and associated OLE, in addition to Phase IIIb and associated LTE), point estimates fluctuate, though the rate of SI per 100 PY by year appears to increase numerically over time (Figure 2)
- In the pooled RMS population, the rate of SI per 100 PY appears to fluctuate over time
- In the PPMS population, the rate of SI per 100 PY by year was higher than in the RMS population
- As observed previously, there was no change in the type or pattern of SI identified by year in patients with RMS or PPMS treated with ocrelizumab, and no pattern was identified with regard to demography, duration of treatment or time following ocrelizumab dosing
Figure 2: Incidence Rates per 100 PY of SI Over Time
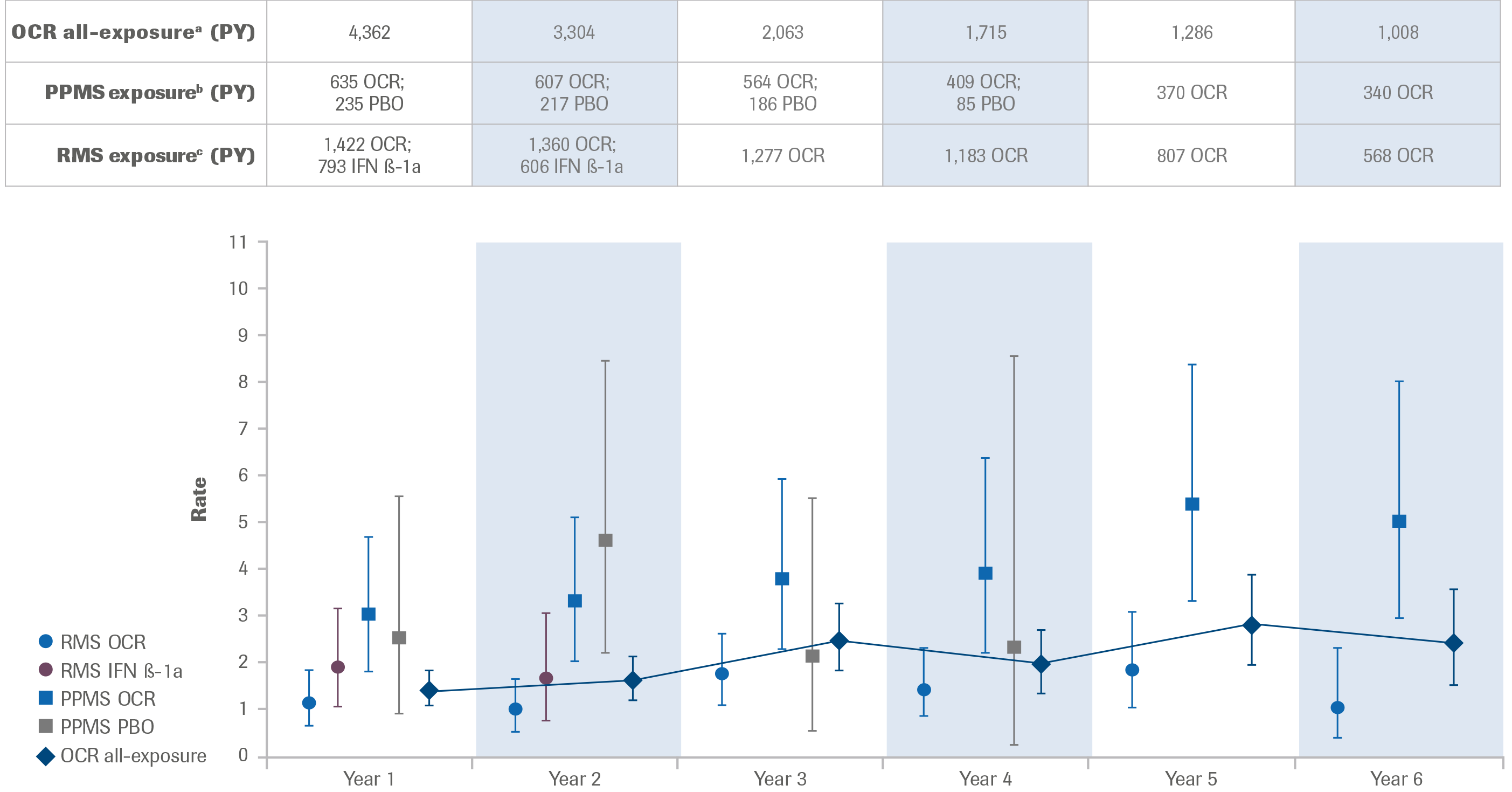
Exposure to ocrelizumab and comparator (IFN β-1a or placebo) in the Phase III pooled RMS population, PPMS population and ocrelizumab all-exposure population in total PY. Investigator text for AEs was encoded using MedDRA versions 18.1 and 21.1. Multiple occurrences of the same AE in one patient are counted multiple times. SIs are defined as serious AEs reported using terms in the MedDRA SOC Infections and infestations. 95% CIs were calculated using an exact method based on the Poisson distribution. Patients are considered in the ongoing year, eg, Year 6 contains patients completing at least 5 years in the study and ongoing during the sixth year.
a Includes patients who received any dose of ocrelizumab during the controlled treatment and associated OLE periods of the Phase II and Phase III studies, plus VELOCE, CHORDS, CASTING (and associated LTE), OBOE and ENSEMBLE.
b Includes patients who received placebo during the controlled treatment period, and any dose of ocrelizumab during the controlled treatment and associated OLE period of the ORATORIO study.
c Includes patients who received IFN β-1a during the controlled treatment period, and any dose of ocrelizumab during the controlled treatment and associated OLE period of the OPERA I and OPERA II studies.
Potential Serious Opportunistic Infections5
- As of January 2019, no additional potential serious opportunistic infections had been reported from ocrelizumab clinical trials since the last data cut-off (July 2018)
- As of January 2019, six potential serious opportunistic infections have been reported from ocrelizumab clinical trials
- Information on confirmed cases of PML in ocrelizumab-treated patients can be found on the PML page of the website
Serum Immunoglobulin Levels6
- At approximately 6 study years of ocrelizumab exposure, a reduction in serum Ig levels was observed at an approximate mean rate of 3–4% per year for IgG, but for the majority of patients Ig levels remain above LLN (Table 1)
- An apparent association between decreased levels of IgG (and less so for IgM or IgA) and SI was observed
- The majority of SIs following episodes of drop in Ig levels <LLN were UTI, cellulitis and pneumonia; most resolved with standard of care, and in most cases patients remained on treatment with ocrelizumab
- This is similar to overall SIs in patients with MS treated with ocrelizumab and is also consistent with types of SIs observed in MS registries
- Most were of Grade 3 (69.1%), none were fatal or opportunistic; most resolved without sequelae (92.6%) within the expected clinical course (78.5% lasted <28 days) by using standard of care treatment, and most resulted in no action taken (dose not changed) with ocrelizumab (87.7%)
Table 1: Rates of SI per 100 PY by IgM, IgG and IgA levels
| Phase III all-exposurea |
IgM | IgG | IgA | ||||
|---|---|---|---|---|---|---|---|
| <LLN | ≥LLN | <LLN | ≥LLN | <LLN |
≥LLN |
||
| Patients (n) | 2,092 | 729 | 1,383 | 152 | 1,940 | 127 | 1,965 |
| Episodes (n) |
– | 929 | 2,368 | 288 | 2,269 | 166 | 2,131 |
| PY | 9,891 | 2,003 | 7,989 | 255 | 9,737 | 256 | 9,726 |
| No. of SIs | 222 | 71 | 151 | 14 | 208 | 7 | 215 |
| Rates of SI per 100 PY | 2.24 | 3.54 | 1.89 | 5.48 | 2.14 | 2.74 | 2.21 |
Post-Marketing Experience4*
- As of 27 March 2019, ~92,037 patients with RMS and PPMS had started ocrelizumab globally outside of clinical trials, corresponding to an exposure of ~80,276 PY
- There were 3,916 events (in 3,050 patients) of infections and infestations reported
- There were 1,535 infections (in 1,227 patients) that were classified as serious
- No new findings related to the type or pattern of SIs were identified
- In these post-marketing case reports, the most commonly reported SIs by preferred terms were UTI and pneumonia, which is in line with clinical trial data
- 35 events had a fatal outcome, corresponding to a rate (95% CI) of 0.04 (0.03, 0.06) per 100 PY. The background rate of fatal SI seen in The Department of Veterans Affairs study was 0.12 (0.08, 0.17) per 100 PY in patients with MS compared with 0.05 (0.03, 0.06) per 100 PY in patients without MS2†
Prescribing information
Indications vary in different countries. The local prescribing information from your country is the primary source of information on the known and potential risks associated with ocrelizumab.
*There are well-recognised limitations that should be considered when interpreting spontaneous post-marketing safety reports, including events may not be causally related to drug exposure; in the real-world setting, events are frequently confounded by factors such as multiple drug use and the presence of pre-existing comorbidities; reporting bias may exist for more significant outcomes, which may result in an over representation of the more serious outcomes; and reporting rates can be stimulated by external factors such as press reports.
†The incidence rates of SI are derived from varied sources, and intended to provide context. Confounding factors that may influence incidence rates have not been accounted for, and therefore, no direct comparisons should be made. Such factors may include, but are not limited to, type of MS, disease duration, risk factors, geographical region, population size, drug exposure, comorbid conditions, treatment history, and duration of follow-up.
The causes of infections are recorded as reported to the company; while the company follows up on all reports to identify the cause, an exact diagnosis is not always possible. Some of the investigations remain ongoing and, therefore, the information may be subject to change.
AE, adverse event; DBP, double-blind period; CI, confidence interval; DMT, disease-modifying treatment; HR, hazard ratio; IFN, interferon; Ig, immunoglobulin; LTE, long-term extension; LLN, lower limit of normal; MedDRA, Medical Dictionary for Regulatory Activities; MS, multiple sclerosis; OCR, ocrelizumab; OLE, open-label extension; PBO, placebo; PML, progressive multifocal leukoencephalopathy; PPMS, primary progressive multiple sclerosis; PY, patient-year; RMS, relapsing multiple sclerosis; SI, serious infection; SOC, system organ class; UTI, urinary tract infection.
- Wijnands JMA, et al. Mult Scler 2017;23:1506–1516;
- Nelson RE, et al. Int J MS Care 2015;17:221–230;
- Wijnands JMA, et al. J Neurol Neurosurg Psychiatry 2018;89:1050–1056;
- Roche data on file;
- Hauser SL, et al. Presented at ECTRIMS 2019 (P648);
- Derfuss T, et al. Presented at ECTRIMS 2019 (Presentation 65)