COVID-19
Data collection
- We are continuously collecting and assessing data from clinical trials, safety surveillance programs, and RWE1,2
COVID-19 in pwMS treated with OCR
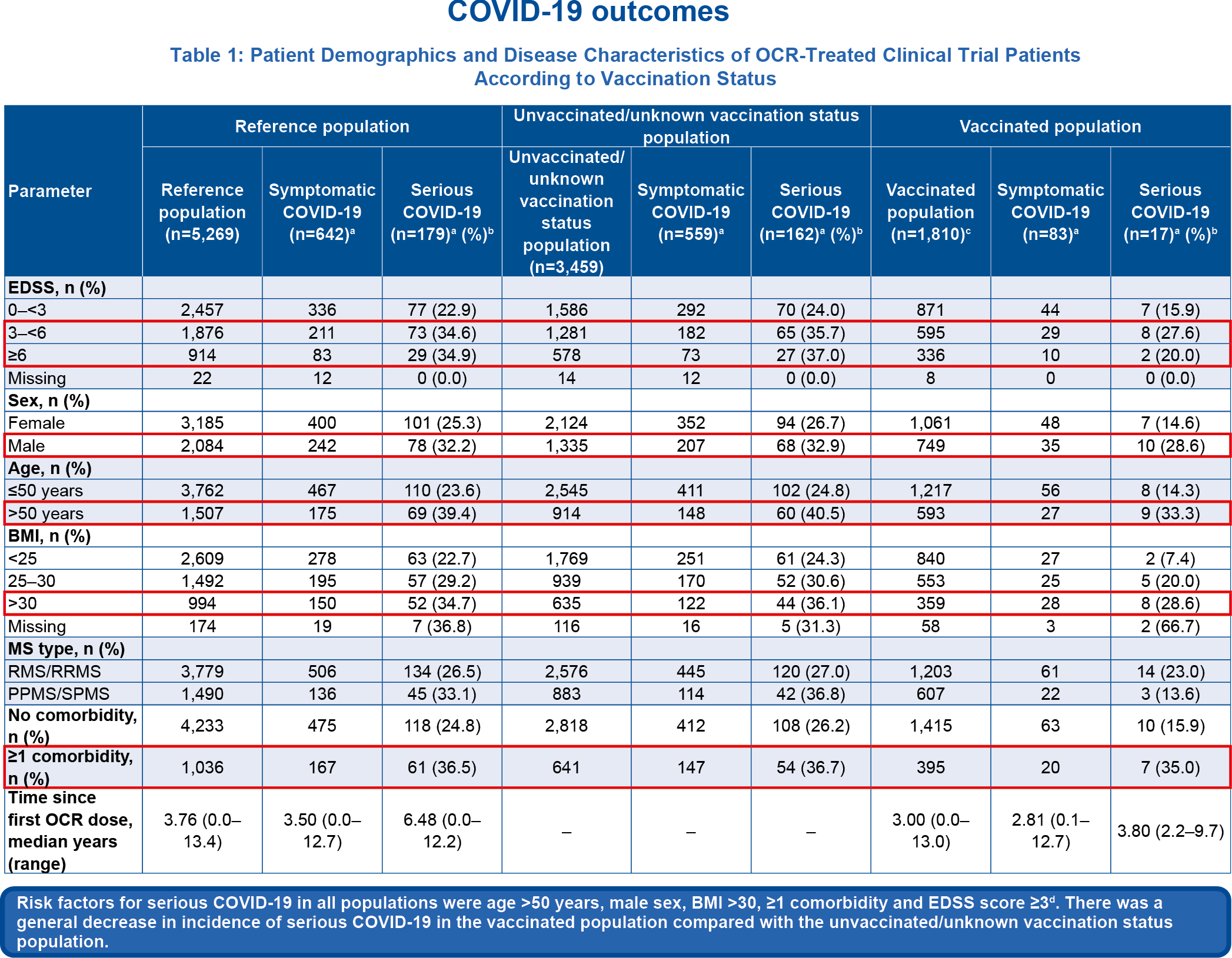
- Vaccination against COVID-19 substantially decreased serious and fatal COVID-19 rates among OCR-treated patients in clinical trials2
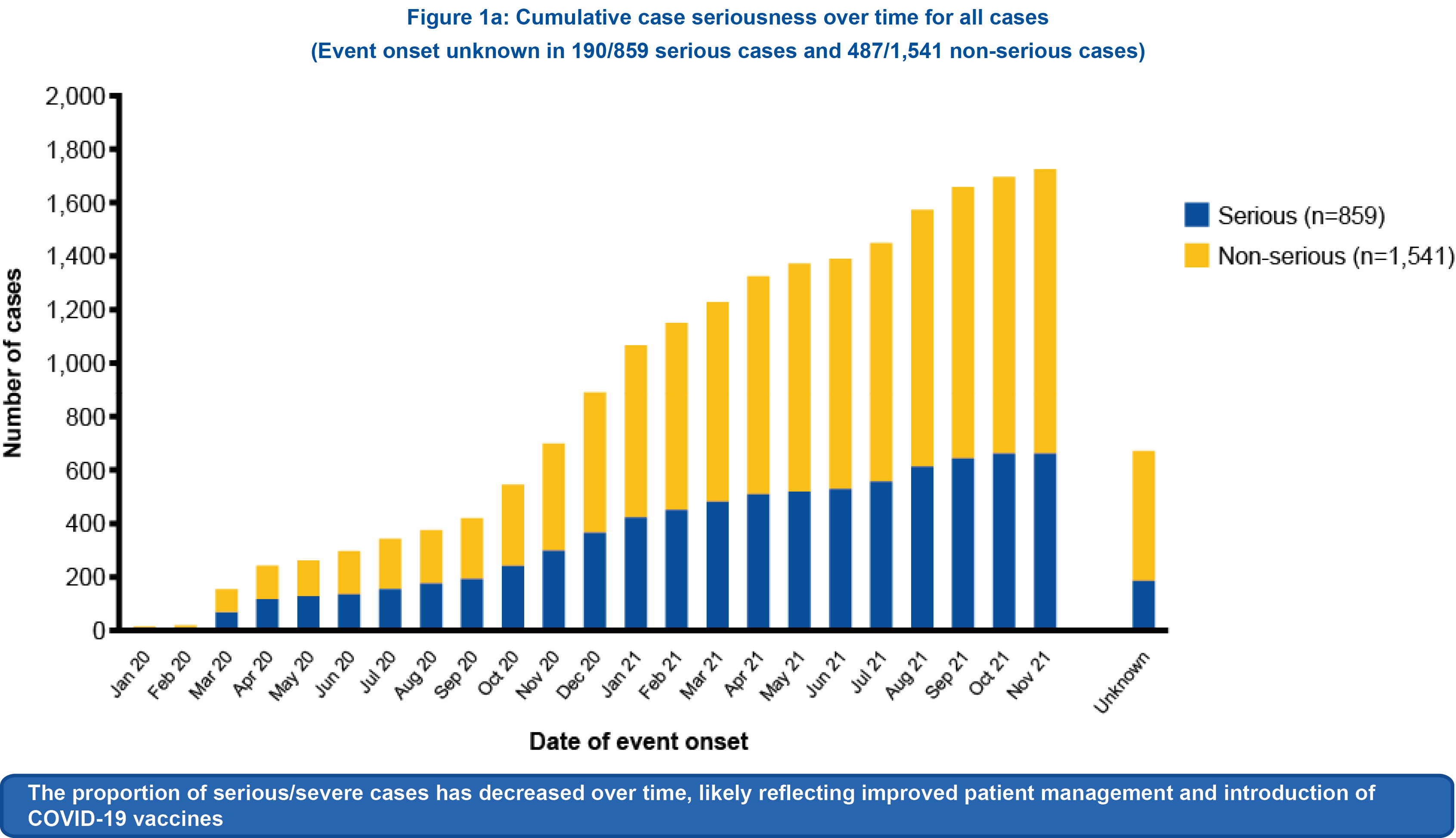
- Serious and fatal cases have declined in the clinical trials population, compared to previous report2–4
- Risk factors for severe COVID-19 have remained the same in vaccinated and unvaccinated ocrelizumab-treated patients2
Latest assessment of COVID-19 data
- As of November 2021:
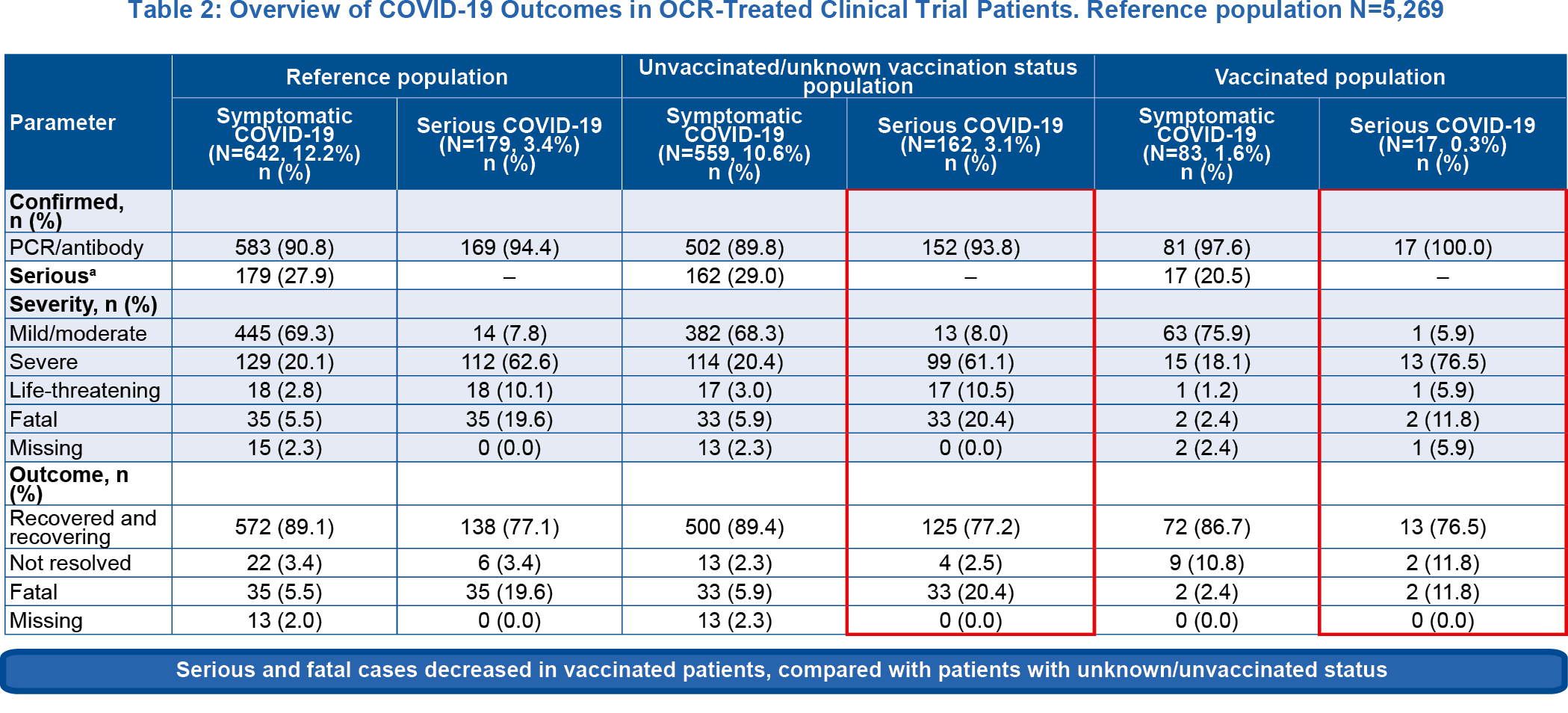
- 642 symptomatic cases of COVID-19 were identified from 5,269 patients in the clinical trials; 463/642 cases were classed as non-serious, with most patients having recovered at the time of the report1,2
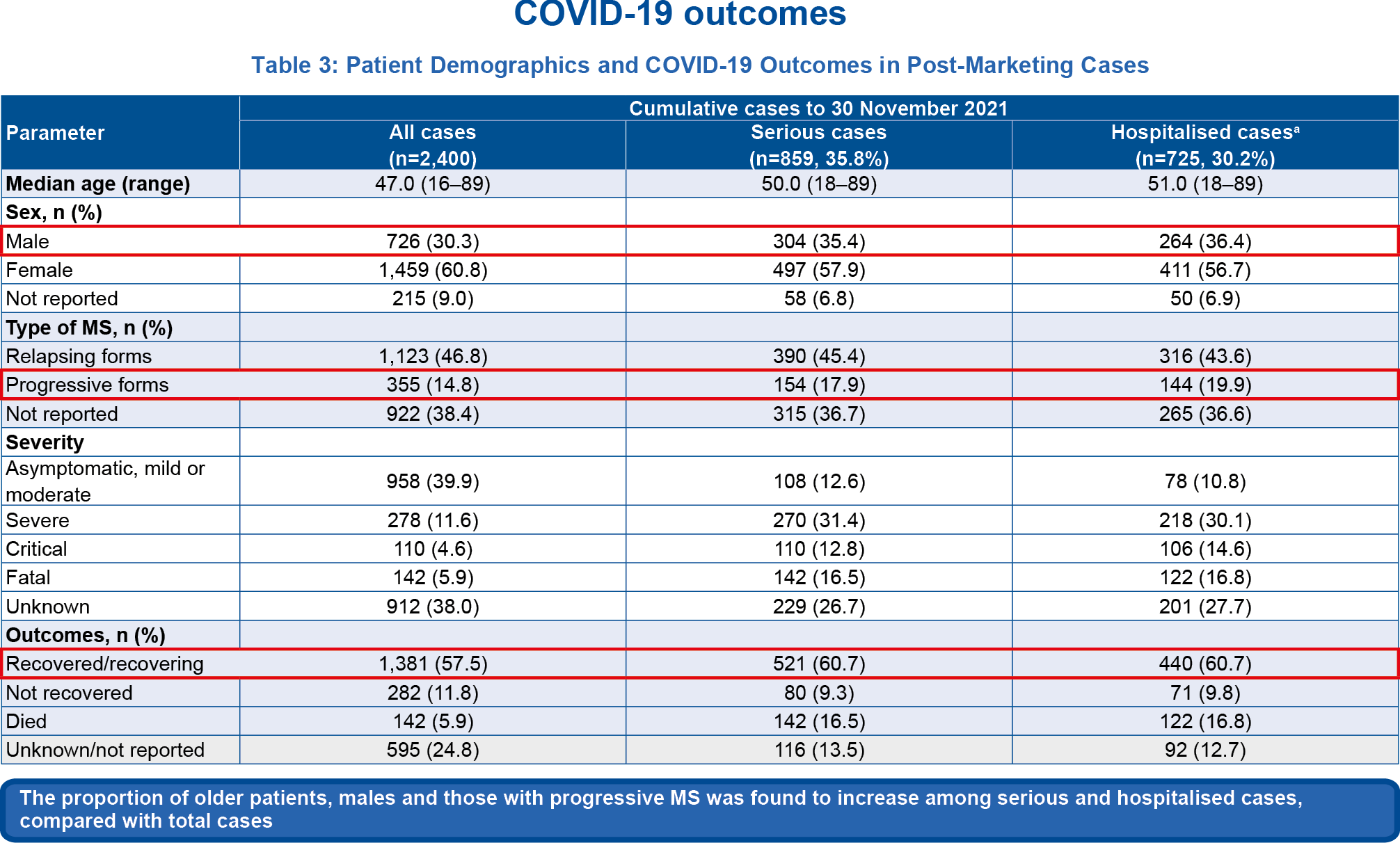
- 2,400 cases were identified in the global safety database with most cases being non-serious 1,541/2,400 (64.2%)1,2
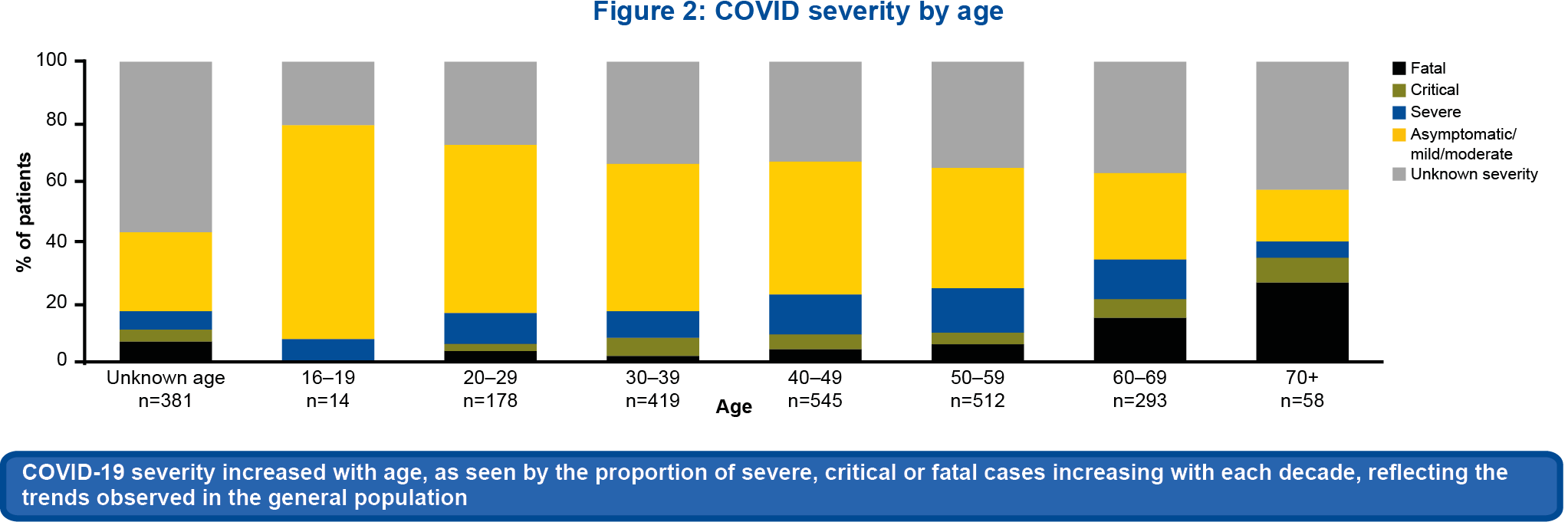
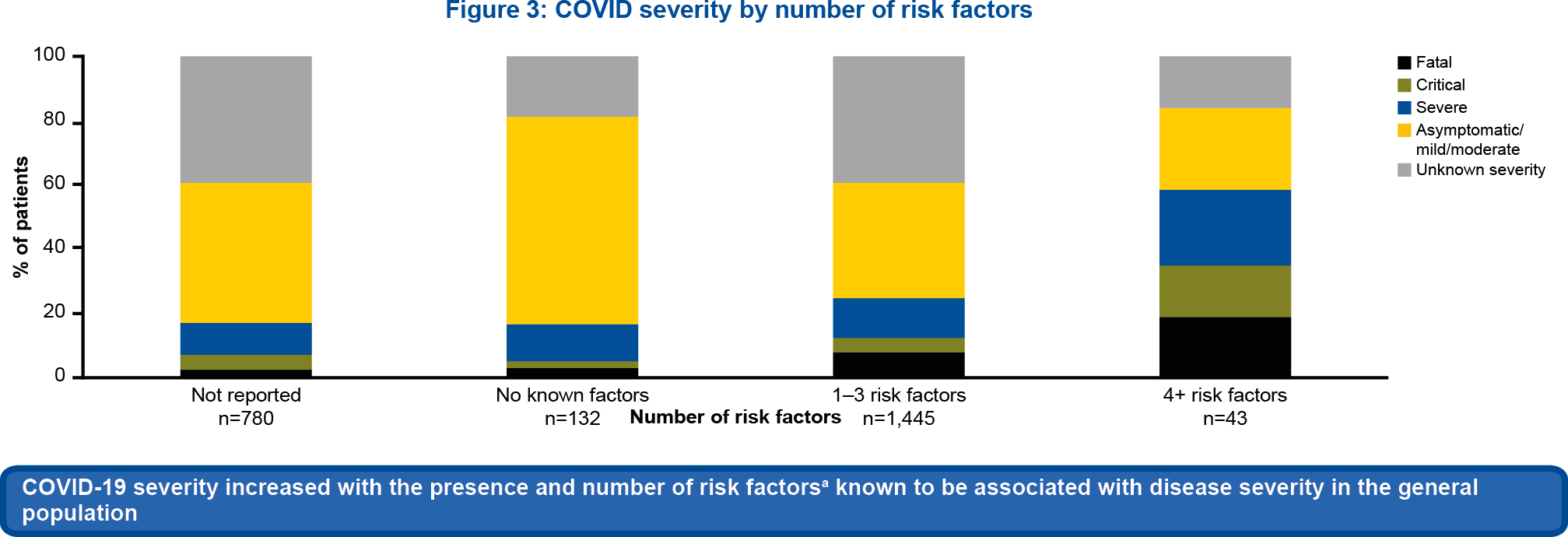
Factors affecting severity of COVID-19
Data Sources
OCR-treated clinical trial patients
Clinical trial data: The reference population refers to the clinical trial population and includes pwMS from 12 ongoing Roche/Genetech clinical trials (OPERA I, OPERA II, ORATORIO, Phase II, LIBERTO, CONSONANCE, ENSEMBLE, VELOCE, OCARINA, OBOE, MUSETTE, GAVOTTE; clinical cut-off date 30 November 2021) who were receiving ongoing OCR treatment since January 2020, with confirmed/unknown/unvaccinated status. Symptomatic cases were captured from this population.
OCR-treated post-marketing patients
OCR-treated pwMS in the Roche/Genetech global safety database.
COVID-19 seriousness
Seriousness of cases was assessed according to the ICH guidelines5
COVID-19 case severity
Clinical trials reported using the CTCAE v5.0 grading system6 : Mild: asymptomatic or mild symptoms; Moderate: minimal, local or non-invasive intervention; Severe: medically significant but not life-threatening; Life-threatening: urgent intervention indicated; Fatal.
For post-marketing reports, assigned as per Hughes et al. (2020)7
Table 1
aMultiple COVID-19 infections in one patient were counted once at the highest severity; bPercentage of serious cases based on symptomatic cases; c271/1,810 vaccinated patients had also received a booster vaccination;.dDescriptive analysis of the baseline characteristics does not allow for any conclusions regarding the cause–effect relationship
*Reported cases were defined as symptomatic, as the vast majority of cases in our database are reported as such and no systematic collection of positive tests in asymptomatic patients has been implemented;
Table 2
aBased on serious event definition of European Medicines Agency, 1995
Table 3
aHospitalised cases are a subset of serious cases.
Figure 1a
2,400 cases were identified in a global safety database as of 30 November 2021; 64.2% (1,541/2,400) were non-serious.
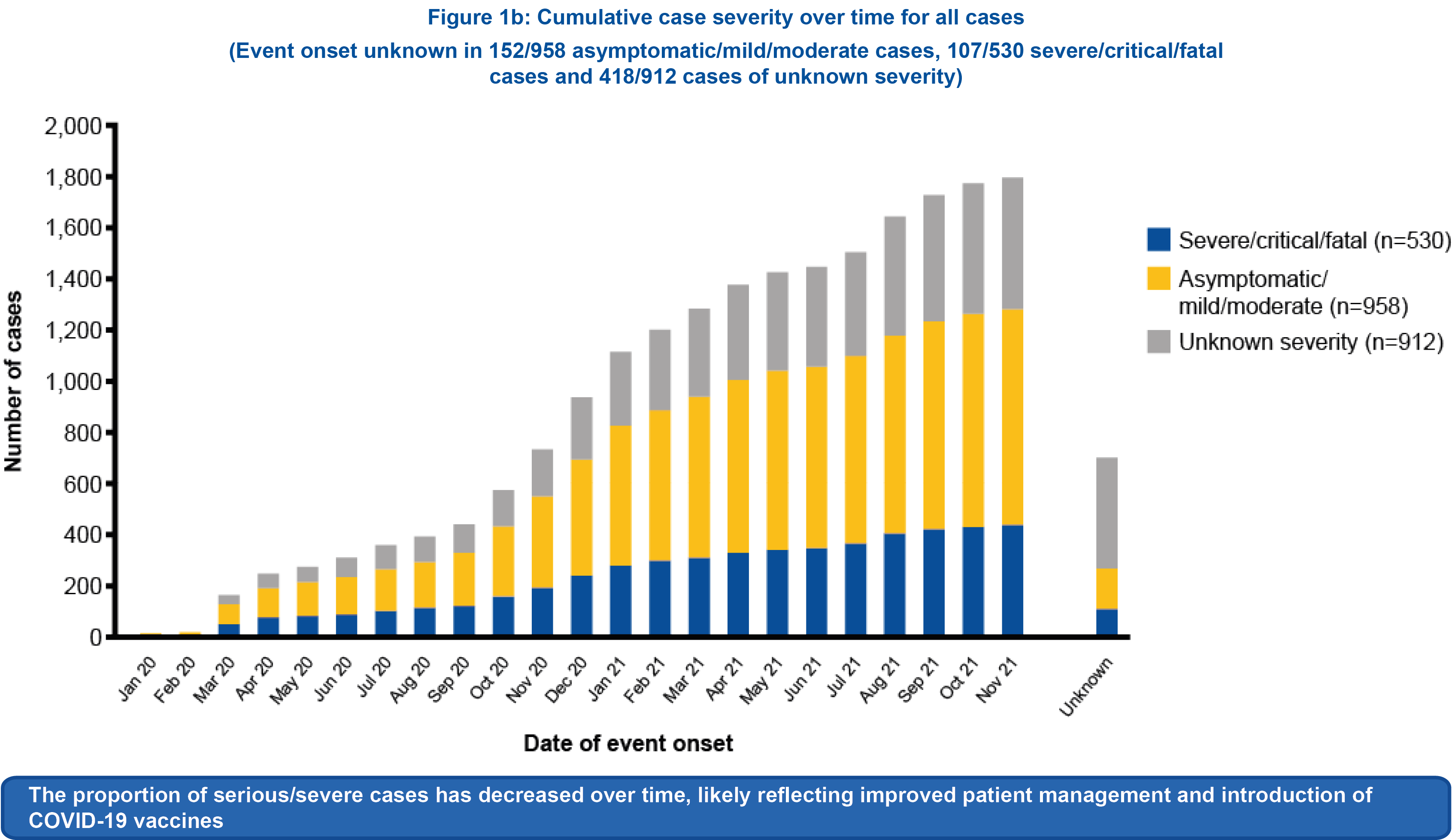
Figure 1b
For cases with sufficient information to assess clinical severity (n=1,488), 64.4% (958/1,488) cases were asymptomatic, mild or moderate.
Figure 2
Although clinical severity could not be determined in 912/2,400 (38.0%) cases due to lack of information, 74.9% (683/912) of these cases were non-serious
Figure 3
aRisk factors for severe COVID-19 include age >50, hypertension, diabetes mellitus, BMI >25, chronic kidney disease, dementia, coronary heart disease, malignancy, chronic pulmonary disease and pregnancy.
Abbreviations
BMI, body mass index; COVID-19, coronavirus disease 2019; COVID, coronavirus disease; CTCAE, Common Terminology Criteria for Adverse Events; EDSS, Expanded Disability Status Scale; ICH, The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use; OCR, ocrelizumab; PCR, polymerase chain reaction; PPMS, primary progressive MS; pwMS, people with MS; PV, pharmacovigilance; RMS, relapsing MS; RRMS, relapsing remitting MS; RWE, real-world evidence; SPMS, secondary progressive MS.
- Roche data on file;
- Hauser SL, et al. Presented at ECTRIMS 2022 (Poster EP1238);
- Hauser SL, et al. ECTRIMS 2021 (Poster P933);
- Richardson S, et al. JAMA 2020;323:2052–9;
- https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use-topic-e-2-clinical-safety-data-management-definitions-and-standards-expedited-reporting-step_en.pdf. Accessed 14 September, 2022;
- U.S. Department of Health and Human Services NIH, NCI. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0., 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Accessed 21 October, 2022;
- Hughes R, et al. Mult Scler Relat Disord 2020;42:102192
M-XX-00012411 (Date of preparation: February 2023)