Ocrelizumab in pregnancy and lactation

Pregnancy outcomes1
- As of 28 March 2024, 3,989 pregnancies had been reported in women with MS treated with OCR
- This is the largest dataset of pregnancy outcomes for an anti-CD20 therapy in MS
- In utero exposure to OCR did not increase the risk of adverse pregnancy or infant outcomes compared with epidemiological background rates of both MS and general populations
- The rates of MCAs were similar in exposed and non-exposed groups, and were in line with other MS cohorts and epidemiologic background rates

MINORE study2–4
- MINORE (NCT04998812) is an ongoing study evaluating placental transfer of OCR in the infants of women with CIS or MS
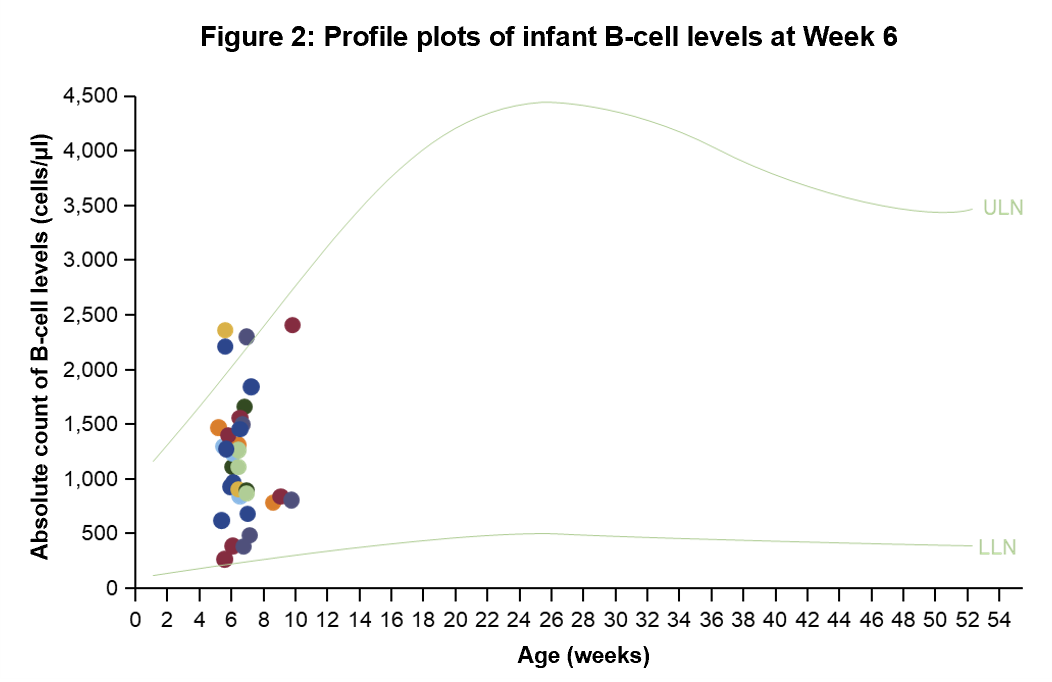
- OCR was undetectable in most umbilical cord serum at birth and infant serum at Week 6 of life and exposure to OCR during pregnancy via placental transfer did not result in infant B-cell depletion
- AEs observed in mothers and infants were typical for pregnancy, delivery, postpartum and infancy

SOPRANINO study4–6
- SOPRANINO (NCT04998851) is an ongoing study evaluating the PK of OCR in the breastmilk of lactating women with CIS or MS
- In infants breastfed by mothers receiving treatment with OCR while breastfeeding, OCR transfer was negligible and B-cell levels were within normal ranges
- Infant health outcomes were consistent with common childhood diseases in the first year of life

Figure 1: Reported pregnancies in women with MS treated with ocrelizumab per year1,7
- The cumulative number of pregnancies reported among women with MS treated with OCR continues to grow with an increase of ~100 new pregnancy cases per month from 2023 to 2024
- Out of the total 3,989 reported pregnancy cases, 3,022 were prospective, while 967 were retrospective or unknown
Cumulative number of OCR pregnancies reported among women with MS
Pregnancies reported by timing of infusion vs exposure: Prospective cases
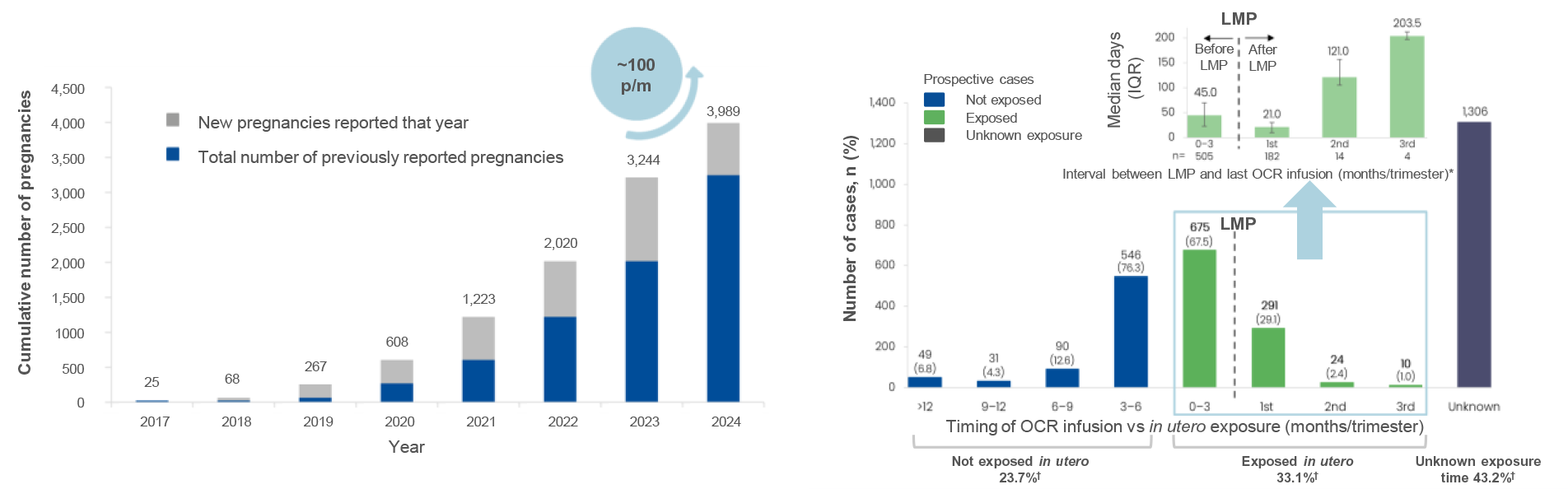
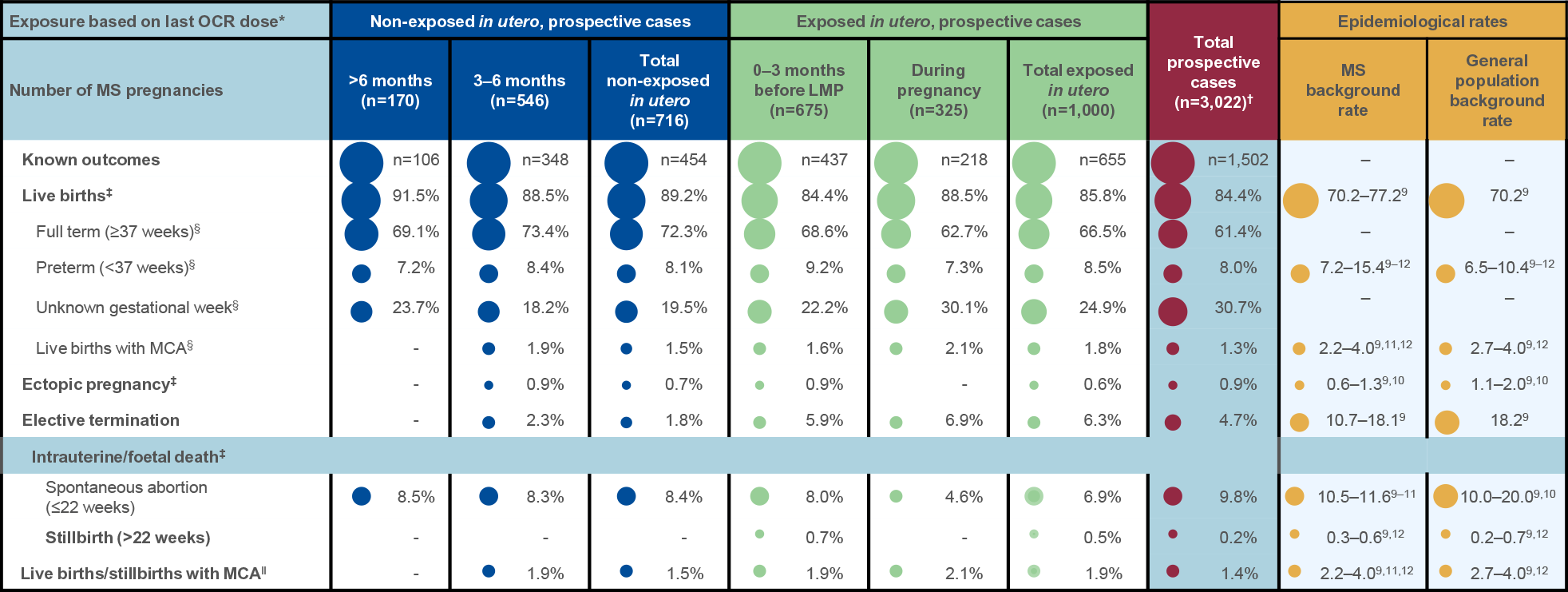
Table 1: Summary of known pregnancy outcomes by exposure category:*
Prospective cases†,1,8
- Most pregnancies resulted in live births (84.4%), and proportions were similar in the exposed and non-exposed groups
- Most live births were full term (61.4%), and a smaller proportion were preterm (8.0%)
- Proportions were similar in the exposed and non-exposed groups
- Gestational age was unknown in 30.7% of live births
- A higher proportion of elective terminations occurred in the exposed group, but the overall cumulative proportion of elective abortions is decreasing (4.7% in 2024 vs 7.4% in 2023 vs 11.5% in 2022 and 15.7% in 2021)
- A smaller proportion of spontaneous abortions occurred in the exposed group (6.9%) compared with the non-exposed group (8.4%)
- The overall rate of stillbirths remained low (0.2%)
Ongoing clinical trials: MINORE & SOPRANINO
MINORE2
- Enrolment of 35 women at ≤GWk 30, whose last OCR dose occurred at any time from 6 months before the LMP until the end of the first trimester
- OCR was undetectable in 32/33 (97.0%) of infant serum samples
- All infant (34/34, 100%) B-cell levels were above the age-specific LLN
- In infants, most AEs were mild to moderate, and the pattern was comparable when stratified for in utero exposure
SOPRANINO6

- Enrolment of 13 women who delivered a full-term infant and made the decision to breastfeed whilst receiving OCR (inclusion from 2–24 weeks postpartum)
- In mothers receiving treatment with OCR while breastfeeding, transfer of OCR into breastmilk was negligible
- OCR levels were undetectable in the serum of breastfed infants and B-cell levels were within normal ranges
- Infant health outcomes were consistent with common childhood diseases in the first year of life
Across both studies, the AEs experienced by mothers were expected as per the established OCR safety profile and/or occurring in postpartum/breastfeeding women as well as in the context of the COVID-19 pandemic2,6
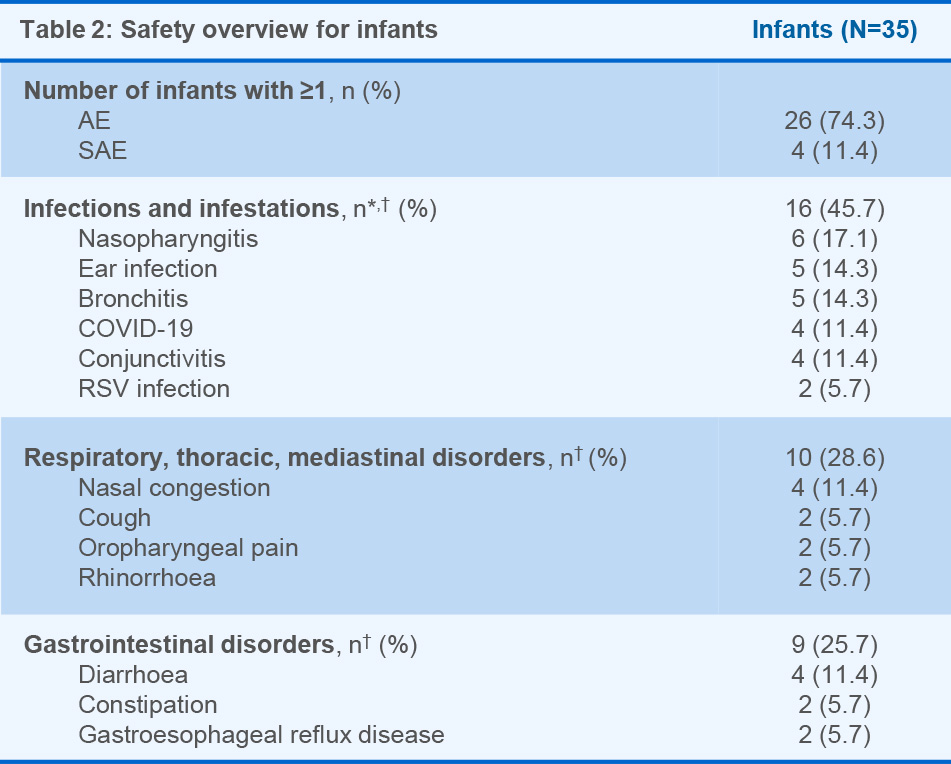
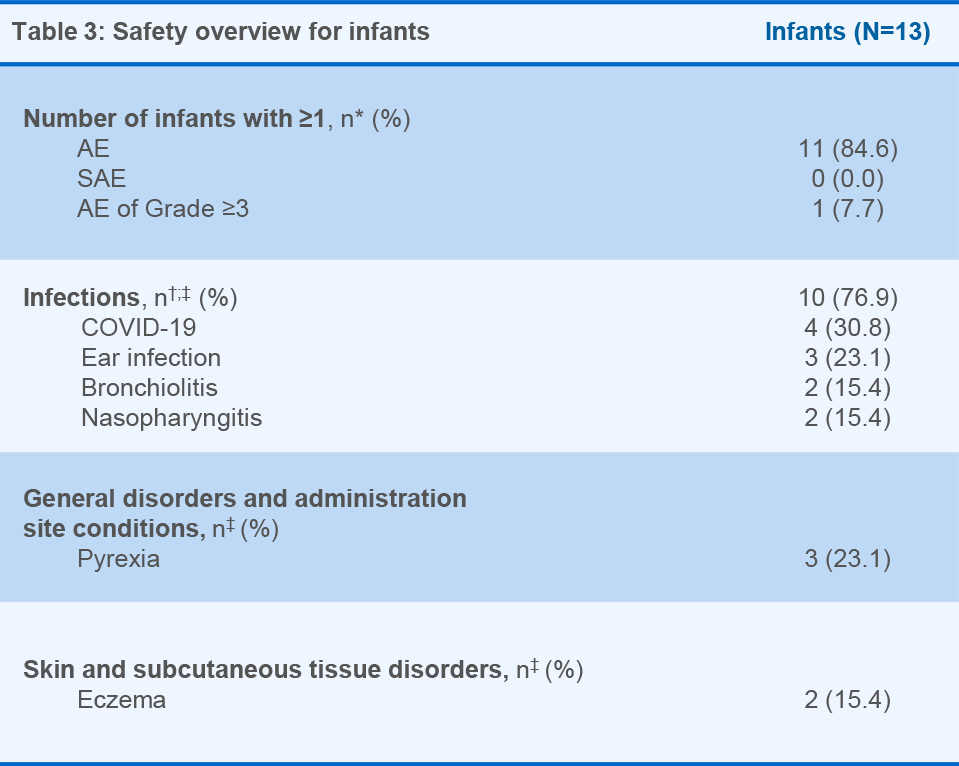
Figure 1: *The interval between LMP and the last OCR infusion was known for 505 women; †Percentages represent fractions of prospective cases with known outcome and known timing of last OCR dose. Table 1: Data as of 28 March, 2024. Dash indicates a data value of 0. *In utero exposure based on timing of last OCR dose relative to the LMP; †Total includes cases of unknown exposure; ‡Percentages represent fractions of the total known outcomes of the respective exposure categories (not exposed in utero, exposed in utero, unknown exposure, total); §Percentages represent fractions of the total live births for the respective exposure categories (not exposed in utero, exposed in utero, unknown exposure, total); ‖Percentages represent fractions of total live births and stillbirths. Figure 2: Normal B-cell ranges in infants are defined by Borriello, et al. 2022.13 Figure 3: Normal B-cell ranges in infants are defined by Borriello, et al. 2022.13 *10/13 infants had B-cell data for the primary analysis; †Dynamic changes occur in the ULN and LLN of B-cell levels throughout the first year. A reduction in B-cell levels is observed from cord blood to the first week of life, followed by a rapid increase over the next 2 months. At ~6 months of age maximum levels are attained, after which levels decrease progressively and stabilise at ~1 year of age; ‡Infants were between 2–24 weeks old at the time of the mother’s first postpartum OCR infusion (corresponding to the WHO and UNICEF’s recommended 6 months of exclusive breastfeeding).14 Table 2: *16 infants presented with a total of 39 infections. Investigator text for AEs encoded using MedDRA version 27.0. Multiple occurrences of the same AE in one individual are counted only once; †Two or more events reported. Table 3: *Based on TEAEs, defined as AEs with onset on or after first dose of study drug administered to the mother, or prior to first dose and end date on or after first dose and with a most extreme NCI CTCAE (version 5.0) grade greater than the initial grade. Investigator text for AEs was encoded using MedDRA version 26.1. Percentages are based on N in the column headings; †10 infants presented with a total of 26 infections. For frequency counts by preferred term, multiple occurrences of the same AE in an individual are counted only once; ‡Two or more events reported. Abbreviations: AE, adverse event; CIS, clinically isolated syndrome; COVID-19, coronavirus disease 2019; CTCAE, Common Terminology Criteria for Adverse Events; GWk, gestation week; Ig, immunoglobulin; IQR, interquartile range; LLN, lower limit of normal; LMP, last menstrual period; MCA, major congenital anomaly; MedDRA, Medical Dictionary for Regulatory Activities; NCI, National Cancer Institute; OCR, ocrelizumab; PK, pharmacokinetics; RSV, respiratory syncytial virus; SAE, serious AE; TEAE, treatment emergent AE; ULN, upper limit of normal; UNICEF, United Nations Children's Fund; WHO, World Health Organization.
Do you have patients with MS receiving OCR who are pregnant? Please remember to report the pregnancy accordingly
Please report any occurrence of pregnancy in women receiving OCR via our MedInfo portal here
References
- Dobson R, et al. Presented at ECTRIMS 2024 (Poster P085);
- Hellwig K, et al. Presented at ECTRIMS 2024 (Poster P087);
- ClinicalTrials.gov identifier: NCT04998812. Accessed 27 September, 2024;
- Bove R, et al. Mult Scler Relat Disord 2022;64:103963;
- ClinicalTrials.gov identifier: NCT04998851. Accessed 27 September, 2024;
- Bove R, et al. Presented at ECTRIMS 2024 (Oral presentation O039);
- Vukusic S, et al. Presented at ECTRIMS 2017 (Poster P710);
- Oreja-Guevara C, et al. Presented at ECTRIMS 2022 (Oral presentation O038);
- Andersen JB, et al. Eur J Neurol 2023;30:162–71;
- Khan E, et al. J Neuroimmunol 2023;383:578178;
- Lopez-Leon S, et al. J Neurol 2020;267:2721–31;
- MacDonald SC, et al. Am J Epidemiol 2019;188:57–66;
- Borriello F, et al. J Allergy Clin Immunol 2022;150:1216–24;
- World Health Organization (WHO). Infant and young child feeding. December 2023. https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding. Accessed 27 September, 2024;
M-XX-00019239 (Date of preparation: January 2025)