Ocrelizumab and malignancies
Clinical trials
- In clinical trials over 11 years, there has been no increased risk of malignancy and female breast cancer with OCR compared with matched reference MS and general populations1–3
Yearly incidence rates
- Cumulative standardised incidence rates of all malignancies and female breast cancer remained within the range reported in registries2–4
Figure 1: Standardised incidence rates per 100 PY and standardised incidence ratio of all malignancies (A) and female breast cancer (B)2


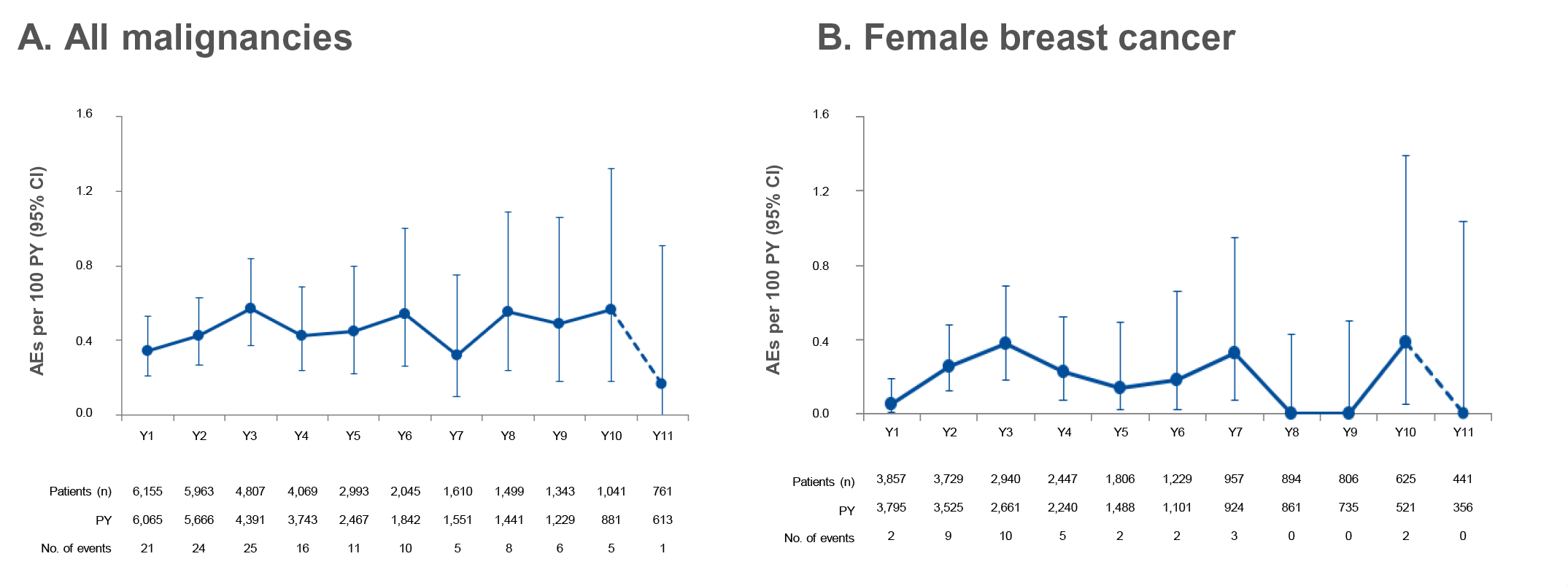
Figure 2: Yearly incidence rates of all malignancies (A) and female breast cancer (B)
in the ocrelizumab all-exposure population*,2
Post-marketing experience*,5
As of March 2024:
A total of 233,196 female patients with RMS and PPMS had started OCR globally outside of RCTs
431 cases of female breast cancer (5 cases were reported in males)
Figure 1: *Standardised incidence rates per 100 PY (95% CI) were reported to allow comparison with the SEER database and the Danish MS Registry, using the direct standardisation method that applies age–sex specific rates to the USA population, with restriction to the age range of the MS clinical trials (15–59 years); †The standardised incidence ratio, calculated as observed/expected number of events, was determined for all malignancies (excluding NMSC) and female breast cancer, using the SEER database and the Danish MS Registry as reference populations; ‡For all malignancies, cases of NMSC were excluded from the MS all-exposure population to allow a comparison with the SEER database; §Includes patients who received any dose of OCR during the CTP and associated OLE periods of the Phase II (NCT00676715) and Phase III (NCT01247324, NCT01412333, NCT01194570) studies plus VELOCE (NCT02545868), CHORDS (NCT02637856), CASTING (NCT02861014), OBOE (NCT02688985), ENSEMBLE (NCT03085810), LIBERTO (NCT03599245), CONSONANCE (NCT03523858), CHIMES (NCT04377555) and OLERO (NCT05269004), including patients originally randomised to comparator (IFN β-1a or PBO) who switched to open-label OCR treatment. Figure 2: *Includes patients who received any dose of OCR during the CTP and associated OLE periods of the Phase II (NCT00676715) and Phase III (NCT01247324, NCT01412333, NCT01194570) studies plus VELOCE (NCT02545868), CHORDS (NCT02637856), CASTING (NCT02861014), OBOE (NCT02688985), ENSEMBLE (NCT03085810), LIBERTO (NCT03599245), CONSONANCE (NCT03523858), CHIMES (NCT04377555) and OLERO (NCT05269004), including patients originally randomised to comparator (IFN β-1a or PBO) who switched to open-label OCR treatment. Post-marketing: *There are well-recognised limitations that should be considered when interpreting spontaneous post-marketing safety reports, including events that may not be causally related to drug exposure; in the real-world setting, events are frequently confounded by factors such as multiple drug use and the presence of pre-existing comorbidities; reporting bias may exist for more significant outcomes, which may result in an overrepresentation of the more serious outcomes; and reporting rates can be stimulated by external factors, such as press reports.The causes of malignancies are recorded as reported to the company; while the company follows up on all reports to identify the cause, an exact diagnosis is not always possible. Some of the investigations remain ongoing and, therefore, the information may be subject to change. PY have been extrapolated from the female to total OCR patients. Case counts from the safety data base had reported at least one of the following AE terms: Breast cancer, invasive papillary breast carcinoma, invasive ductal breast carcinoma, breast cancer female, HER2 positive breast cancer, intraductal proliferative breast lesion, breast cancer recurrent, breast cancer stage I, breast cancer stage II, invasive breast carcinoma, breast cancer in situ, breast cancer metastatic, breast cancer stage III, breast cancer stage IV, breast neoplasm, hormone receptor positive breast cancer, invasive lobular breast carcinoma, lobular breast carcinoma in situ, triple negative breast cancer. Abbreviations: AE, adverse event; CI, confidence interval; CTP, controlled treatment period; HER2, human epidermal growth factor receptor 2; IFN β-1a, interferon beta-1a; NMSC, non-melanoma skin cancer; OCR, ocrelizumab; OLE, open-label extension; PBO, placebo; PPMS, primary progressive MS; PY, patient-years; RCT, randomised controlled trial; RMS, relapsing MS; SEER, Surveillance, Epidemiology, and End Results Program; Y, year.
Indications vary in different countries. The local prescribing information from your country is the primary source of information on the known and potential risks associated with ocrelizumab.
References
- Hauser SL, et al. Neurology 2021;97:e1546–59;
- Hauser SL, et al. Presented at ECTRIMS 2024 (Poster P300);
- Nørgaard M, et al. Mult Scler Relat Disord 2019;28:81–5;
- National Institutes of Health (NIH). Available at https://seer.cancer.gov. Accessed 27 September, 2024;
- Roche data on file.
M-XX-00019230 (Date of preparation: December 2024)