Progressive multifocal leukoencephalopathy (PML)

- As of May 2025 there have been 18 confirmed cases of PML in more than 420,000 patients treated with ocrelizumab globally, amounting to a total of more than 1,200,000 patient years (in clinical trials and post-marketing use). Of these, 12 were carry-over* cases attributed to a prior DMT1-3
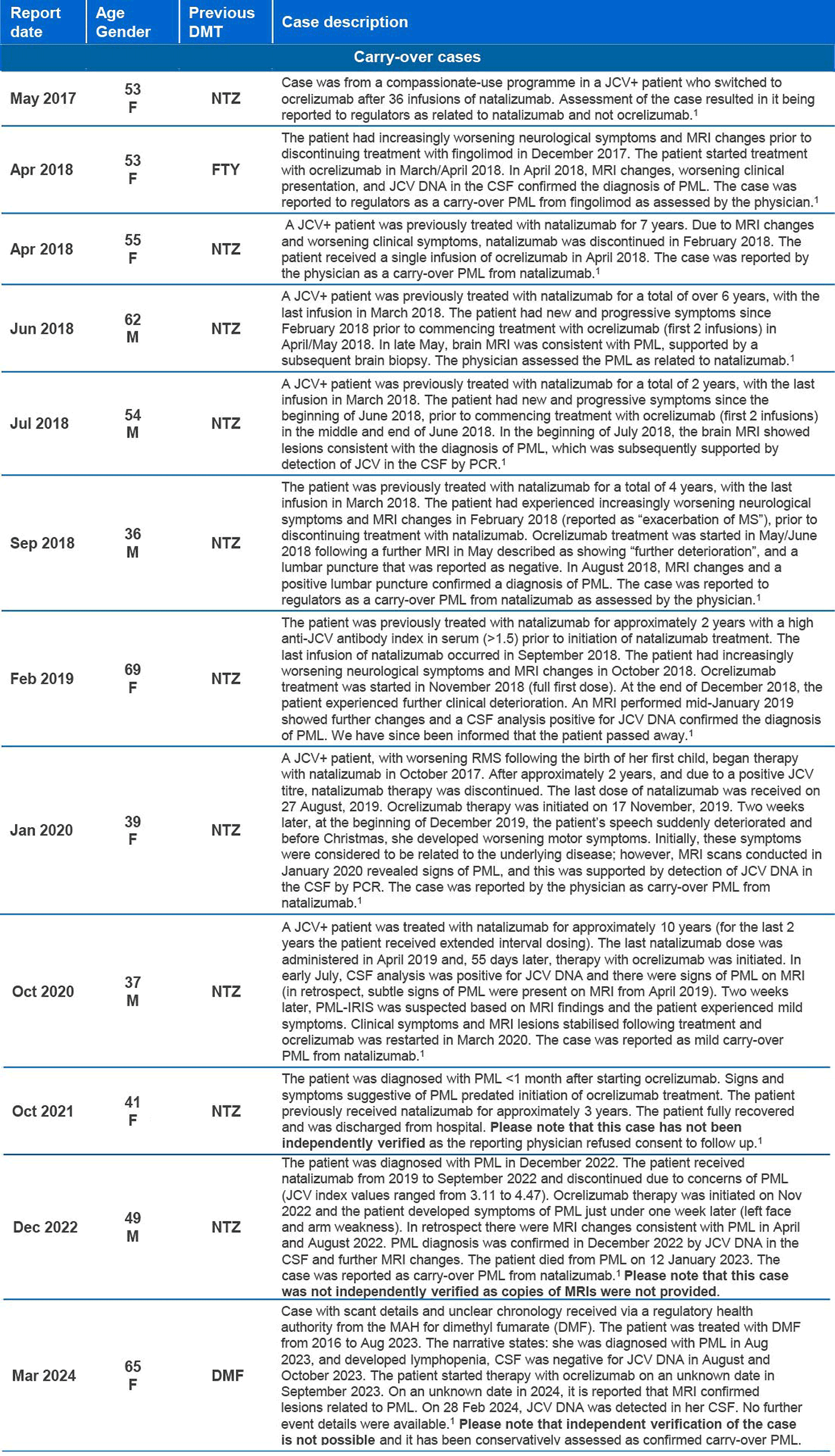
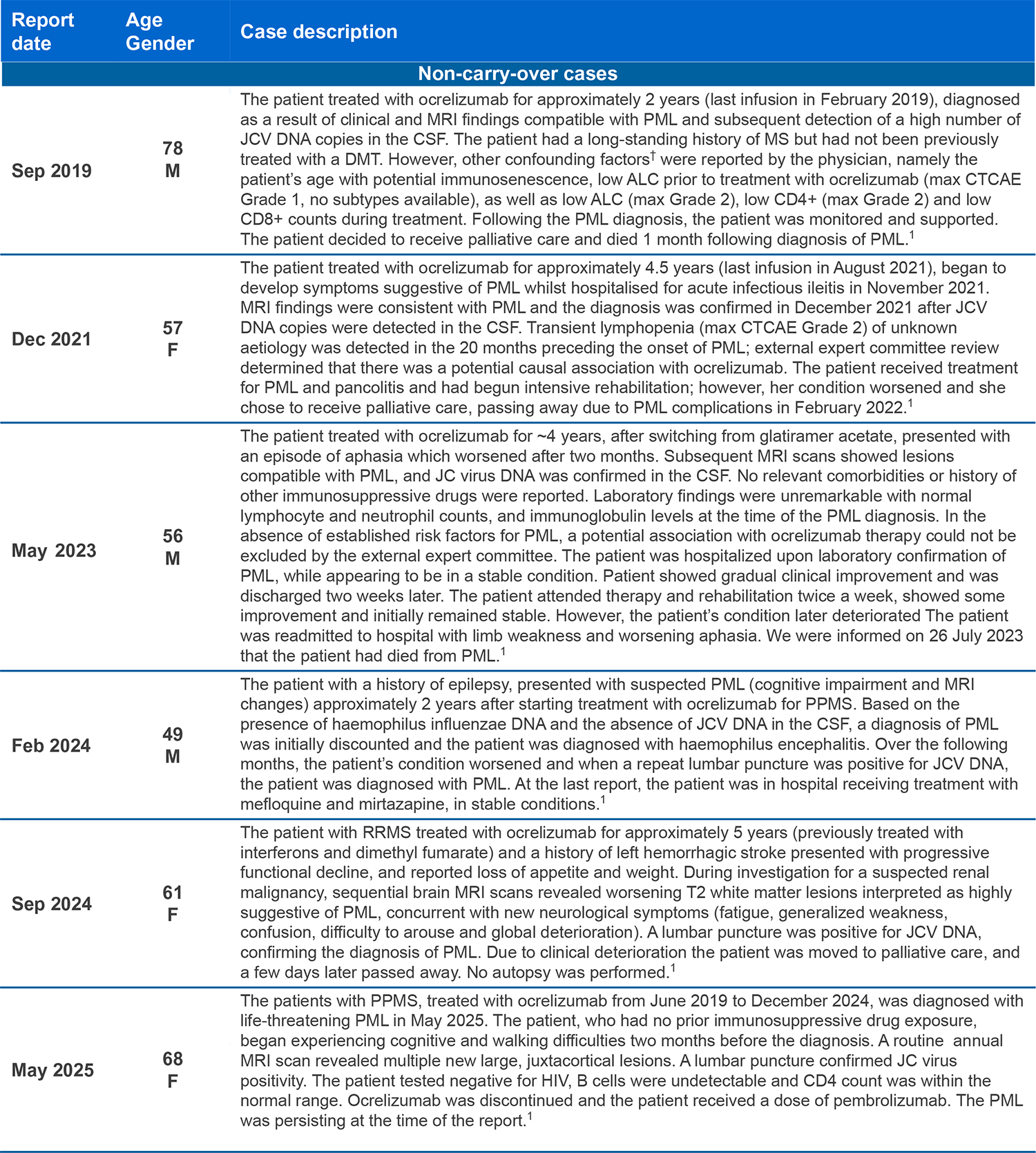
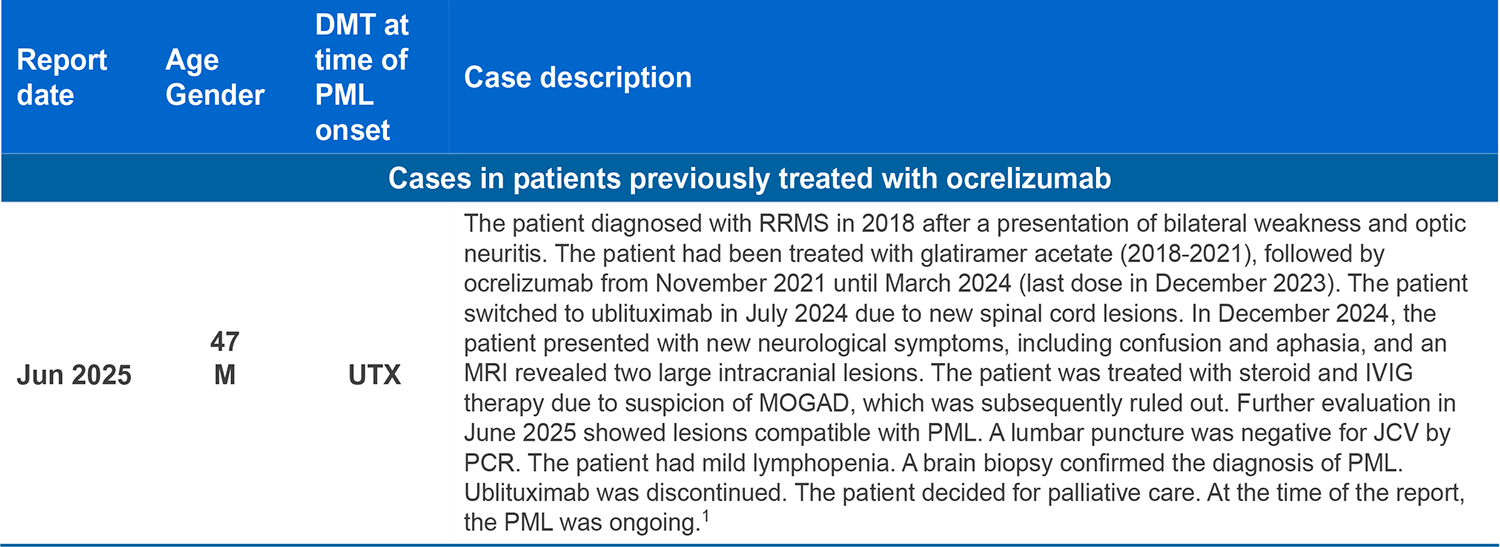
*Carry-over PML: PML that develops a few months after stopping one DMT known to increase the risk of PML and starting a different DMT. In these cases, PML could have developed without causing symptoms while the patient was still on the previous DMT, or shortly after stopping the previous DMT;4
†Confounding of adverse event reporting occurs when the assessment of association between exposure to a drug and an adverse event is distorted by the effect of one or several other variables that are also risk factors for the outcome of interest;5,6 in the cases detailed above, confounders included factors such prior treatment with another DMT (carry-over PML), age-related immunosenescence and lymphopenia.
Abbreviations
ALC, absolute lymphocyte count; COVID-19, coronavirus disease 2019; CSF, cerebrospinal fluid; CTCAE, Common Terminology Criteria for Adverse Events; DMF, dimethyl fumarate; DMT, disease-modifying therapy; DNA, deoxyribonucleic acid; FTY, fingolimod; JCV, John Cunningham virus; MAH, marketing authorisation holder; MOGAD, Myelin oligodendrocyte glycoprotein antibody-associated disease; NTZ, natalizumab; PCR, polymerase chain reaction; PML, progressive multifocal leukoencephalopathy; PML-IRIS, PML immune reconstitution inflammatory syndrome; PPMS, primary progressive MS; RMS, relapsing MS; RRMS, relapsing remitting MS, UTX, ublituximab.
Progressive Multifocal Leukoencephalopathy (PML) is an opportunistic viral infection of the brain caused by the John Cunningham (JC) virus that typically only occurs in patients who are immunocompromised, and that usually leads to death or severe disability.
Although no cases of PML were identified in ocrelizumab clinical trials, a risk of PML cannot be ruled out since JC virus infection resulting in PML has been observed in patients treated with anti-CD20 antibodies and other MS therapies and associated with risk factors (e.g., patient population, polytherapy with immunosuppressants). Physicians should be vigilant for the early signs and symptoms of PML, which can include any new onset, or worsening of neurological signs or symptoms, as these can be similar to MS disease.
If PML is suspected, dosing with ocrelizumab must be withheld. Evaluation including MRI scan, preferably with contrast (compared with pre-treatment MRI), confirmatory CSF testing for JC viral DNA and repeat neurological assessments should be considered. If PML is confirmed, treatment must be discontinued permanently.
When switching from drugs with prolonged immune effects, such as fingolimod, natalizumab, teriflunomide, cladribine or mitoxantrone, consider the duration and mode of action of these drugs because of additive immunosuppressive effects when initiating ocrelizumab.1,2
*Indications vary in different countries. The local prescribing information for your country is the primary source of information on the known and potential risks associated with ocrelizumab.
References
- Roche data on file;
- Roche data on file, Ocrelizumab Cumulative Multiple Sclerosis Patient Exposure clinical trials and post-marketing experience data cut-off Mar 2025
- Clifford DB, et al. Presented at ECTRIMS 2019 (Poster P970);
- Giovannoni G, et al. Pract Neurol 2016;16:389–93;
- Varallo FR, et al. Clin Ther 2017;39:686–96;
- Mills EA, Mao-Draayer Y. Mult Scler 2018;24:1014–22.